Hsv Vaccine Trials 2021
Friedman and Weissman are on track to begin human clinical trials of their HSV-2 mRNA vaccine in 2022. The company dedicated to discovering vaccines for herpes is back in the news.

Harvey M Friedman S Research Works University Of Pennsylvania Pa Up And Other Places
The need for a herpes vaccine is clear.

Hsv vaccine trials 2021. Actual Study Start Date. Herpes Vaccine Candidates. There are two different types of herpes virus.
Vaccine efficacy was 20 95 confidence interval CI -29 to 50 against genital herpes disease. The disease can mainly occur around mouth or genitals and also on different parts of the body. Overall the vaccine was not efficacious.
Genital herpes vaccine candidate heads for human studies. THURSDAY June 17 2021 HealthDay News -- Aiming to deliver a one-two punch to the herpes virus animal research on an experimental drug found it tackled active infections and reduced or. CAMBRIDGE Mass March 30 2021 PRNewswire -- Two novel vaccine candidates for the prevention and treatment of genital herpes have shown to be safe in pre-clinical animal studies according to a.
HSV Vaccines Currently in Clinical Trials Vaccine Company Candidate Adjuvant Current Phase Results Synthetic peptide complex with HHSP 70 Agenus HerpV 32 peptides QS-21 II therapeutic 17 reduction in shedding 75 reduction in viral load Recombinant subunit Genocea GEN-003 ICP4 gD2 Matrix-M2 II therapeutic 60 reduction in. RVx201 is a first-in-human HSV-2 live-attenuated therapeutic vaccine that is modified to be interferon sensitive and destroy the ability of the virus to inhibit immune responses. New Herpes Vaccine Shows 9367 Efficiency To Cure HSV-1 HSV-2.
Sydney Australia July 07 2020 GLOBE NEWSWIRE -- The race is on to find a vaccine for COVID-19 the illness caused by the. Martine Aubert left and Keith Jerome of the Fred Hutchinson Cancer Research Center led one of the teams that won STAT Madness. All STDs are different so this exact vaccine for HSV-2 wouldnt protect against other STDs but Friedman believes that once targets are identified for other specific STDs mRNA may be the best way to develop an effective vaccine.
A Phase I Single-blind Randomised Placebo-controlled Dose Escalation Study to Evaluate the Reactogenicity Safety and Immune Response of an HSV Vaccine in Healthy Participants Aged 18-40 Years. April 5 2021. The vaccine developed by a team led by University of Pennsylvania researcher Harvey Friedman has been shown in.
GlaxoSmithKline GSK launched a limited phase 1 clinical trial for a herpes simplex virus type 2 HSV-2 candidate known as HSV vaccine GSK4108771A. HSV529 HSV15 is a vaccine candidate. This USA-based study will include sixty participants and was last updated on March 18 2021.
To describe the safety profile of different investigational vaccine regimens against herpes simplex virus type 2 HSV-2. Kitts in the Caribbean using live attenuated virus on volunteers who were suffering from herpes simplex. Two herpes vaccine candidates RVx201 and RVx202 have been shown to be safe in a new pre-clinical study by researchers at the Department of Population Health Sciences Virginia Tech and the University of Oklahoma Health Sciences Center.
MarketQuestbiz has declared a new market research study entitled Global Herpes Simplex Virus HSV Vaccines Market 2021 by Manufacturers Regions Type and Application Forecast to 2026 that comprises information with respect to the significant occasions that have occurred or are presently occurring in the business space. When challenged with a virulent strain of the sexually transmitted HSV-2. A genetically edited form of a herpes simplex virus rewired to keep it from taking refuge in the nervous system and eluding an immune response has outperformed a leading vaccine candidate in a new study from the University of Cincinnati Northwestern University and the University of NebraskaLincoln.
June 17 2021 iCrowdNewswire. Actual Study Completion Date. Clin Microbiol Infect.
If you are interested in joining a clinical trial ask your doctor about any trials open in your area or contact hospitals andor universities to find out about clinical trials available for you. The report explains the current state. 2021 Last Verified.
Actual Primary Completion Date. Delta gD-2 gD-2 is a vaccine candidate based on an HSV-2 virus genetically deleted for glycoprotein D gD-2. A genetically edited form of a herpes simplex virus has outperformed a leading vaccine candidate in a new study.
The HSV vaccine was associated with an increased risk of local reactions as compared with the control vaccine and it elicited ELISA and neutralizing antibodies to HSV-2. Fred Hutchinson Cancer Research Center. Hopes that a vaccine against genital herpes may finally be on the horizon after decades of failed RD have been rekindled by a new candidate heading for clinical trials.
In recent years there have been growing cases of herpes across the globe. Top Companies Analysis Business Demand Cost structure SWOT analysis. Bill Halford bypassed FDA protocol for vaccine development and set up a small trial on the island of St.
The Pfizer BNT162b2 vaccine showed a reassuring safety profile in clinical trials but real-world data are scarce. Lack of coverage has left millions infected with herpes in the dark. Herpes simplex virus HSV vaccines help preventing the occurrence of herpes disease.
History of HSV infection of the eye eg herpes simplex interstitial keratitis or uveitis. Fernandez explained that the herpes simplex virus has evolved to evade the immune system downregulating its first line of defense the interferon response. Press Release Herpes Simplex Virus HSV Vaccines Market Growth Overview with Top Countries Detailed Analysis 2021-2027.
Online ahead of printABSTRACTOBJECTIVE. Herpes clinical trials are carried out to test new treatments that could possibly cure the disease and to create and test vaccines to prevent infection. This first-time-in-human study intends to assess the reactogenicity safety and immunogenicity of four different.
Previous vaccination against HSV infection with either the trial vaccine or another vaccine against HSV. Posted November 10 2020 by Scott Schrage. About half a billion people worldwide between the ages of 15-49 have genital herpes infection caused by either HSV-1 or HSV-2 according to the World Health Organization WHOIn the United States alone an estimated 1 in 6 adults have genital herpes with around 300000 new infections diagnosed each year.
Rational Vaccines gained notoriety when its founder the late Dr. Herpes vaccines in clinical trials include therapeutic intended to reduce viral shedding in people already infected with HSV and preventive vaccine candidates.

Cutaneous Reactions After Sars Cov 2 Vaccination A Cross Sectional Spanish Nationwide Study Of 405 Cases Catala British Journal Of Dermatology Wiley Online Library

Katalin Kariko Kkariko Twitter

Potential Mechanisms Of Anaphylaxis To Covid 19 Mrna Vaccines Journal Of Allergy And Clinical Immunology
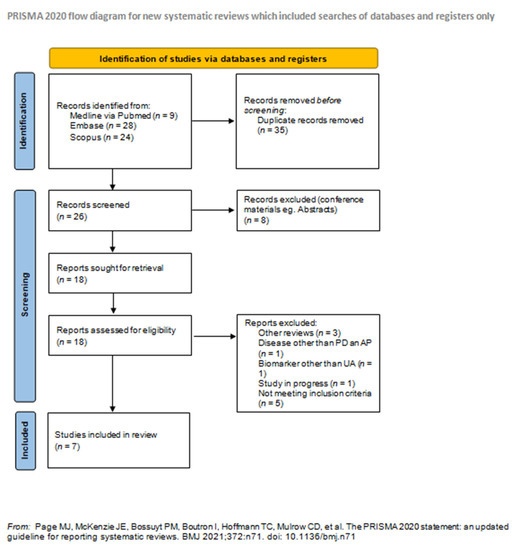
Medicina September 2021 Browse Articles

China S Sinopharm Debuts New Vaccines Targeting Covid 19 Variants Youtube

Harvey M Friedman S Research Works University Of Pennsylvania Pa Up And Other Places

Harvey M Friedman S Research Works University Of Pennsylvania Pa Up And Other Places

Potential Mechanisms Of Anaphylaxis To Covid 19 Mrna Vaccines Journal Of Allergy And Clinical Immunology

Harvey M Friedman S Research Works University Of Pennsylvania Pa Up And Other Places

Harvey M Friedman S Research Works University Of Pennsylvania Pa Up And Other Places

Harvey M Friedman S Research Works University Of Pennsylvania Pa Up And Other Places
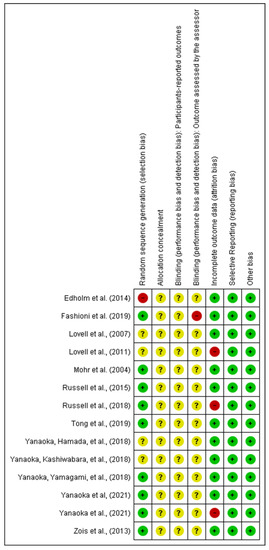
Medicina September 2021 Browse Articles

Harvey M Friedman S Research Works University Of Pennsylvania Pa Up And Other Places
Posting Komentar untuk "Hsv Vaccine Trials 2021"